Antibody Development Services
At Vertebrate Antibodies Ltd (VAL), we offer comprehensive antibody development services, including hybridoma development, polyclonal antibody production, and nanobody generation. Our expert scientists combine academic insight with industrial precision to deliver antibodies that meet the highest standards of specificity and performance.
Antibody type
Beyond hybridoma technology, we also specialize in custom polyclonal antibody production and nanobody engineering. These services give researchers access to diverse binding formats, enabling more flexible assay design and novel diagnostic or therapeutic strategies.
Vertebrate Antibodies Ltd helps researchers accelerate discovery while maintaining full control over antibody specificity, affinity, and reproducibility. Whether your project is academic, diagnostic, or biotechnological, VAL delivers dependable results that support scientific innovation.
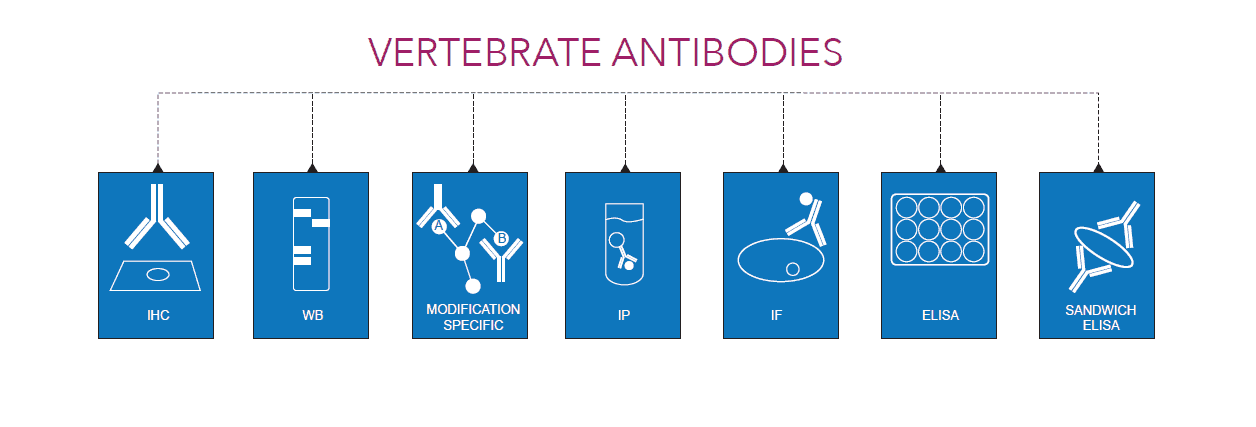
Applications
As part of our commitment to the global research community, VAL provides a low-risk, antibody development package for a variety of applications, such as: Western Blot (WB), Immunohistochemistry (IHC), Enzyme-Linked Immunosorbent Assay (ELISA) and Immunofluorescence (IF).
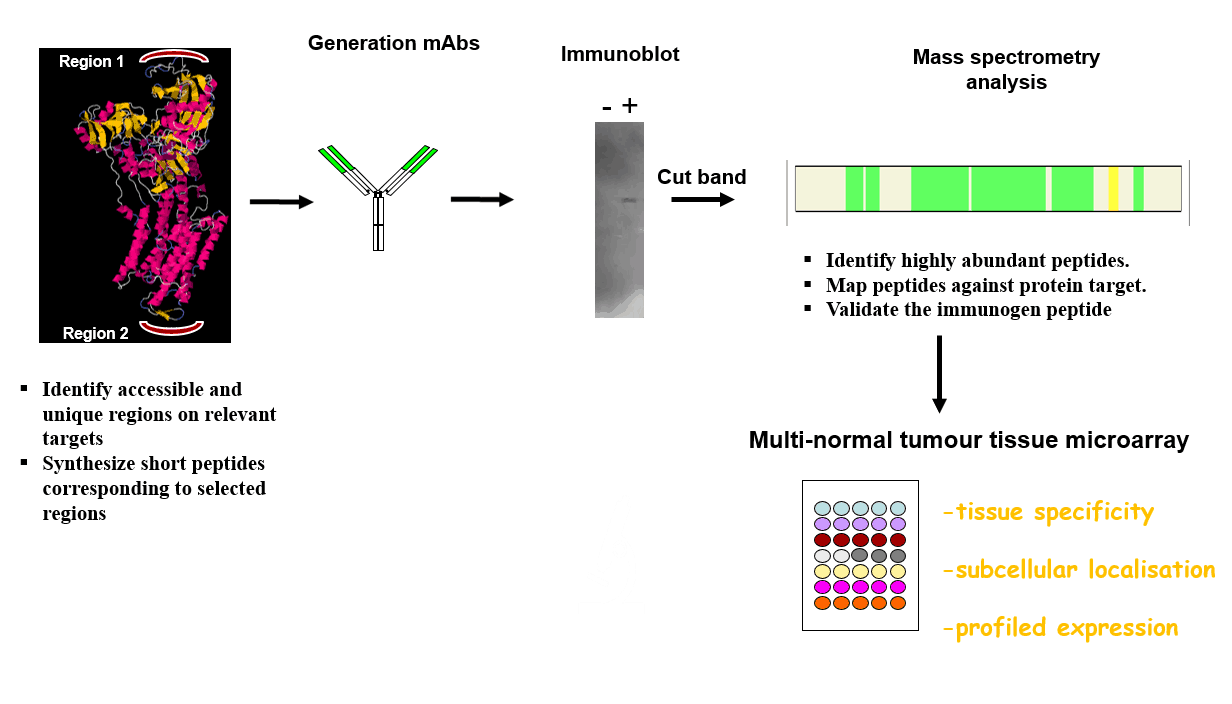
VAL hybridoma development service content
- ANTIGEN DESIGN: Using bioinformatics, VAL will assist with the selection of the peptide antigen, identifying exposed, hydrophilic, flexible and immunogenic sites.
- PEPTIDE SYNTHESIS & CONJUGATION: Peptide synthesis (at least 80% purity, quality control by HPLC, MS) and conjugation to the desired protein carriers.
- IMMUNISATION: Standard immunization according to a schedule over 42 days.
- CELL FUSION: Splenocytes with best serum antibody response are fused with myeloma cell line.
- HYBRIDOMA SELECTION AND SCREENING: Hybrid cells selection (HAT) and culture supernatant screening vs. target antigen.
- EXPANSION OF BEST CLONES:
- Hybridoma cell lines subcloned to monoclonality & tested in the selected assay systems
- Final antibodies selected and characterised.
- Clones are (i) supplied to sponsor (ii) frozen and banked at VAL premises as back-up. Thereafter the sponsor may elect to have antibody produced/purified by VAL.