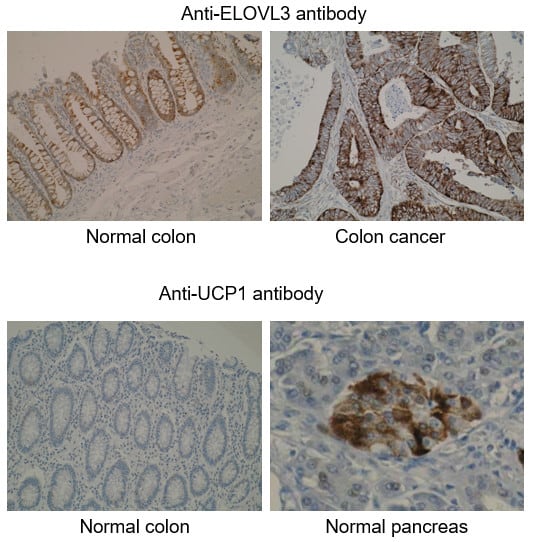
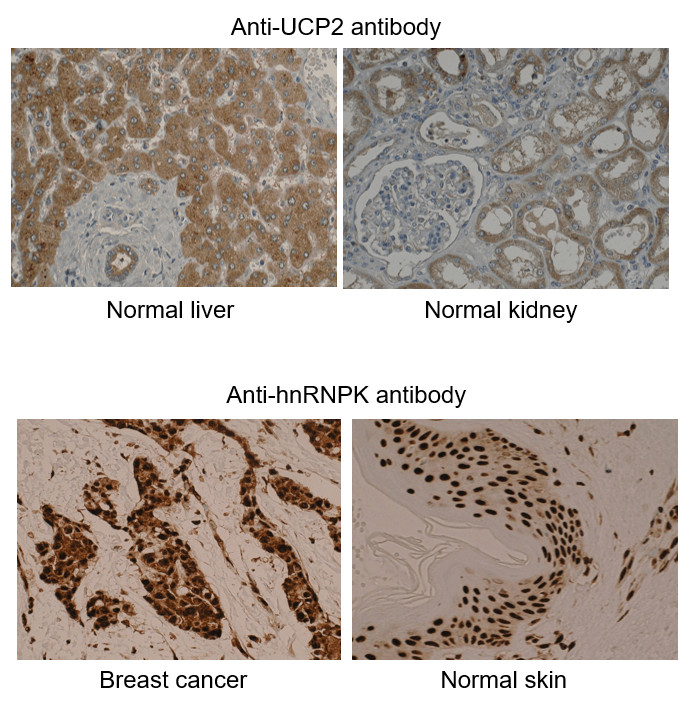
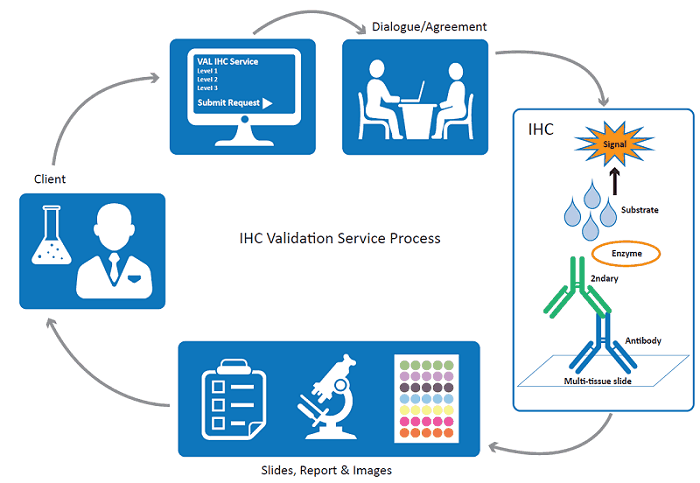
What is IHC Validation?
Immunohistochemistry (IHC) is the process of using antibodies to detect specific antigens within a tissue section. Providing a wealth of information regarding target abundance and localization, it is widely used to compare normal and diseased tissue samples. This highlights its importance to support a diversity of research and clinical applications.
Meaningful IHC data depends on sensitive and highly specific antibodies. Poor sensitivity can result in a low abundance target going undetected, while off target binding can produce unwanted background signal, leading to misinterpretation of IHC staining. To avoid these issues and assure confidence in IHC results, researchers require antibodies which have been fully characterized, validated, and optimised for IHC use.
Why choose VAL for IHC validation of your antibody?
As part of our commitment to support the scientific community by providing high quality antibodies to advance research, we offer a complete service package to accurately evaluate antibody specificity and suitability for IHC. The IHC service will be performed in a NQUAS (National Quality Assurance Standards) accredited laboratory that is externally assessed. Using Dako IHC stainer (Autostainer Link 48), all antibodies will be validated on a comprehensive multi-tissue microarray, comprising normal (n=53) and tumour (n=72) tissues from 20 different major organs. The immunoreactivity and tissue specificity of antibodies will be assessed by consultant pathologist. Important note: all tissue specimen were received fresh in the diagnostic histopathology laboratory where they were reviewed and areas of tissue to be sampled were first identified and marked on the appropriate haematoxylin and eosin stained slide by an expert pathologist.
- Antigen retrieval conditions
- Recommended primary antibody dilution
- Cellular localisation.
- Tissue and cellular specificity
- High quality antibody staining images (typically images of four different tissues, although specific tissue images may be requested)
- IHC protocol
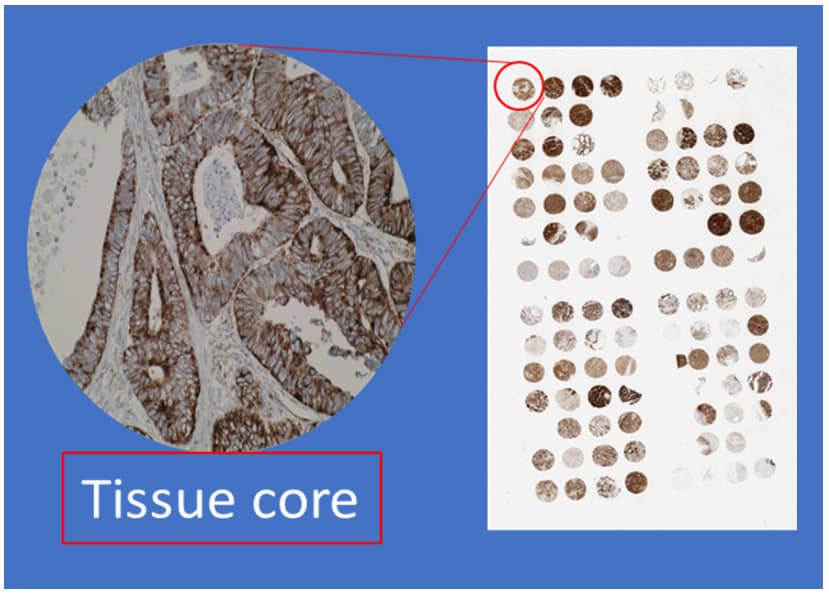
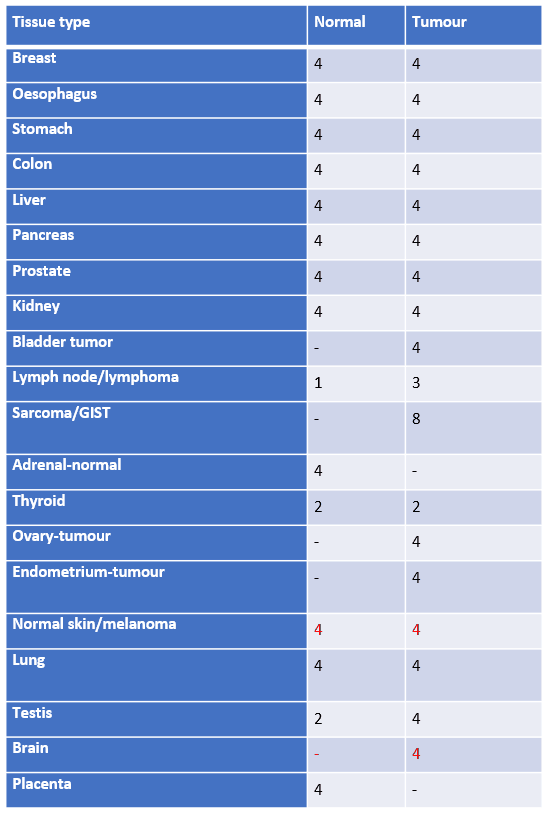
Our multi-tissue microarray comprises 53 normal and 72 tumour tissues from 20 different major organs to deliver comprehensive IHC validation of your antibody.
Our IHC validation portfolio is tiered to accommodate varying customer needs. As well as offering IHC antibody characterisation against our multi-tissue microarray, we provide services for antibodies with unknown antigen retrieval conditions, unknown optimal dilutions, or an unknown expression profile. Competitively priced, these are typically delivered in a turnaround time of just 3-5 weeks.
Services can be added on to one of our custom antibody production packages for a complete end-to-end solution. Alternatively, you can supply your own mouse or rabbit antibody for assessment by our highly specialised team. Working with a purified antibody or supernatant, we offer a range of deliverables to meet the exacting requirements of your project.
Level 1: IHC-basic
Providing researchers with extra confidence in the performance of antibodies which have already been validated and optimised for IHC, our level 1 IHC validation service involves thorough IHC antibody characterisation against our multi-tissue microarray.
To benefit from this service, we ask that you provide enough primary antibody for us to use 400-600µl of the final antibody dilution per slide. This should be accompanied by detailed information regarding the antigen retrieval conditions and optimal antibody working concentration/dilution, and recommendations for appropriate positive and negative control tissues.
In return, we will deliver one of the following:
- Summary of staining results and high-quality images of stained tissues
- CZI file containing whole slide scans using the Zeiss scanner
- Cover-slipped stained slides
Level 2: IHC-validation
Intended to provide validation of uncharacterised antibodies using our multi-tissue microarray, our level 2 IHC validation service involves testing each antibody under three different sets of pre-treatment conditions. This generates three slides per antibody, delivering detailed information regarding antibody quality and specificity.
Like our level 1 service, we require 400-600µl of the final antibody dilution per slide. Having performed our evaluation, we will deliver a full report by a consultant pathologist detailing antigen retrieval conditions, cellular localisation, tissue/cellular specificity and antibody quality, and one of the following:
- High-quality images of stained tissues
- CZI file containing whole slide scans using the Zeiss scanner
- Cover-slipped stained slides
Level 3: IHC-validation-optimisation
Our highest tier, level 3 IHC validation service provides validation and optimisation of uncharacterised antibodies on our multi-tissue microarray using three different sets of pre-treatment conditions and three dilutions of each antibody.
Antibody requirements for this service are of course greater to account for the increased number of variables. We ask that you provide enough primary antibody for us to use 400-600µl of the final antibody dilution per slide.
Deliverables for our level 3 service include a full report by a consultant pathologist detailing antigen retrieval conditions, optimal antibody working concentration/dilution, cellular localisation, tissue/cellular specificity and antibody quality. This is accompanied by one of the following:
- High-quality images of stained tissues
- A CZI file containing whole slide scans using the Zeiss scanner
- Cover-slipped stained slides
At VAL, we understand that every antibody is different. This means we recognise the importance of validating every antibody in the application within which it will be used. Our IHC validation service assures confidence in antibody IHC performance, empowering researchers to generate high quality IHC data to drive discovery.
To find out more about VAL’s IHC Validation service, please get in touch with the VAL team.
Example IHC Images